Pipeline
HCW Biologics is a clinical-stage company advancing a pipeline of potentially transformative immunotherapeutic product candidates for the treatment of diseases promoted by chronic inflammation, especially age-related diseases, including those diseases that diminish quality of life.
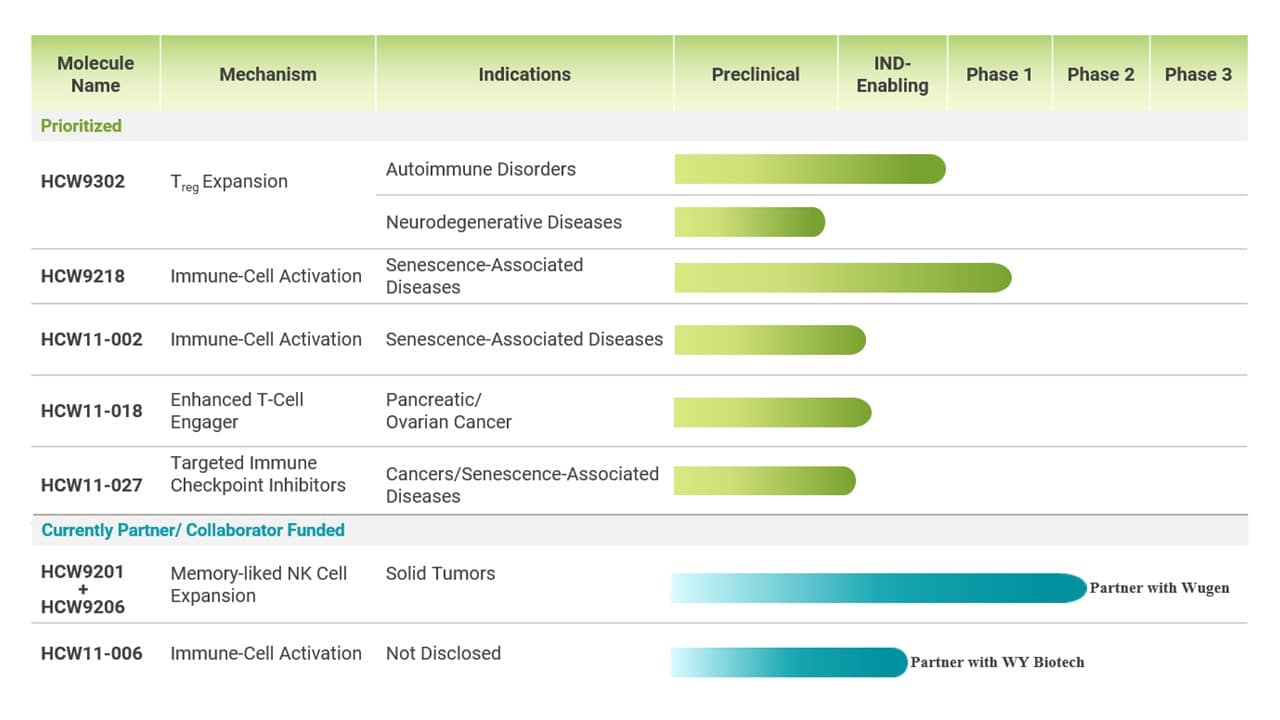
NOTES TO CLINICAL PIPELINE:
- As a result of the settlement agreement and release HCWB entered into with ImmunityBio on July 13, 2024 (“Settlement Agreement”), the Company retains perpetual, exclusive worldwide rights to develop immunotherapeutic treatments based on HCW9218 for aging-related diseases other than cancer. The Company is currently assessing potential indications that are suitable to advance in investigative studies.
- The Company has ownership of the Wugen License between the Company and Wugen entered into in 2020. One of Wugen’s lead clinical programs, WU-NK-101, is based on HCWB licensed molecules. Wugen holds an exclusive worldwide license for two of our molecules, HCW9201 and HCW9206. The Wugen License conveys limited rights to develop cell-based therapy treatments for cancer using the licensed molecules. HCW Biologics has retained all other rights to HCW9201 and HCW9206, including, but not limited to, manufacturing rights and injectable rights.
HCW9302 Program
Our lead molecule, HCW9302, is an injectable single-chain, IL-2-based fusion protein. Preclinical studies in mouse models have demonstrated the ability of HCW9302 to activate Treg cells and reduce inflammation-related diseases, supporting the potential of HCW9302 to treat a wide variety of autoimmune and age-related diseases. The Company has submitted an Investigational New Drug application (“IND”) to the FDA for a Phase 1 clinical trial to evaluate HCW9302 in an autoimmune indication. The Company has also identified clinical investigators and sites interested in conducting this study once the FDA issues the letter to proceed.
HCW9218 Program
HCW9218, is an injectable, bifunctional fusion protein complex that innovatively combines a TGF-β receptor to neutralize a highly immunosuppressive cytokine secreted by tumors, and IL-15, a potent cytokine, to stimulate natural killer (“NK”) cells and CD8+ T cell cytotoxicity.
A Phase 1 dose escalation clinical trial (NCT05322408) of subcutaneous HCW9218 in patients with chemotherapy-refractory solid tumor cancers has been completed at the Masonic Cancer Center, University of Minnesota. A total of 18 patients participated in this study, with over 66% of patients with ovarian cancer (4/6) showing evidence of stable disease after receiving HCW9218 at 0.5 mg/kg or greater. While two dose-limiting toxicities (“DLTs”) were experienced, neither of these events triggered treatment stopping rules. The most frequent treatment-related adverse events (at least possibly related to the study medication) were injections site reactions, flu-like symptoms and decreased lymphocyte counts, all of which are consistent with previous clinical experience with IL-15-based therapies. Stimulation of NK and CD8+ T cell responses and neutralization of TGF-β were also observed in patients, consistent with the results of preclinical studies. This trial met its primary endpoints, including determination the Recommended Phase 2 Dose (“RP2D”) for future clinical development.
While the Company does not plan to pursue further use of HCW9218 in cancer indications, future clinical development of HCW9218 in other indications will employ the subcutaneous RP2D and treatment schedule defined in its oncology clinical studies. The Company is currently assessing a number of indications broadly categorized as “quality of life” – including aesthetics for deep wrinkles and senile lentigo – in search of candidates to advance HCW9218 in clinical development.
HCW9206 Program
HCW9206 is a fusion protein complex created with the TOBI platform based on common gamma chain (γc) cytokines IL-7, IL-15 and IL-21, which are known to play important roles in NK cell and T cell homeostasis. We hold the rights to the injectable form of HCW9206, and ex vivo rights are licensed to Wugen. This molecule is designed to broadly activate the immune system to support expansion of NK cells and T cells ex vivo and in vivo.
We are currently in the discovery stage of HCW9206, which is to create an injectable immunotherapeutic. We believe our preclinical testing of HCW9206 has demonstrated that this complex can support infused NK cells for a long duration and enable NK cells to sustain cytotoxicity against tumor cells. In addition, we are currently conducting preclinical studies to investigate the use of HCW9206 to enhance vaccine efficacy in different vaccine models as well as to support CAR-T cells in vivo.
TRBC Programs
We have developed a second-generation drug discovery technology, the TRBC platform, to design immunotherapeutics that not only activate and target immune responses but are also equipped with receptors that specifically target cancerous or infected cells. Further preclinical evaluation studies are currently being conducted for three molecules we have selected based on promising early data. These include HCW11-002 which we are evaluating in senescence-associated diseases, the second-generation immune checkpoint inhibitor we call HCW11-0027, and immune cell engagers – HCW11-018.
Other Programs
Part of the Company’s strategy is to license marketing rights and other rights that are outside of our core focus.
In 2020, we entered into an Exclusive Worldwide License Agreement with Wugen Inc. for two molecules constructed with our TOBI platform, for the rights to use these molecules to develop cell-based therapies for cancer. Part of the Company’s upfront license fee was paid in Wugen common shares, which currently represent a 5.6% ownership interest in Wugen. The Company retained manufacturing rights for the license molecules.
In 2024, we entered into a License, Research and Co-Development Agreement with WY Biotech for one of our new proprietary preclinical molecules which was built with TRBC platform technology. The Company has an opportunity to reclaim the rights to some regions after a Phase 1 clinical study is completed.
The Company continues to evaluate its portfolio to identify compounds which may be good candidates for licensing or other collaborations, where it will be to our advantage to leverage the expertise and financial strength of a partner in the development of a compound.

