Our Focus
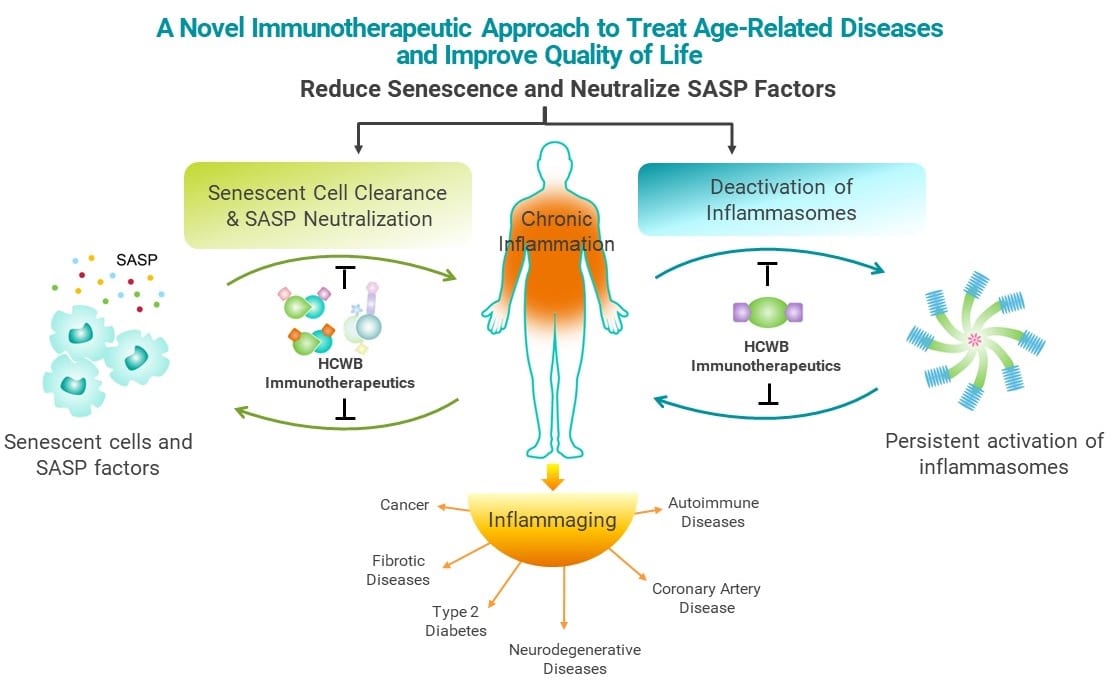
While inflammation is part of the normal repair response for healing, when it becomes prolonged and persists, it is damaging and destructive. Several common molecular pathways have been identified that are associated chronic low-grade inflammation, especially common as we age. Our view is that there are two primary underlying processes that drive chronic inflammation: Accumulation of senescent cells and activation of inflammasomes. Both of these processes generate pro-inflammatory factors that drive senescence.
Senescent cells remain metabolically active and can influence tissue hemostasis, disease, and aging through their senescence-associated secretory phenotype (“SASP”) factors. Senescence is considered to be a physiologic process and is important in promoting wound healing, tissue homeostasis, regeneration, and regulation of fibrosis. Senescence also plays a role in tumor suppression. The accumulation of senescent cells, due to the aging of our immune cells, drives aging and age-related diseases and conditions. The SASP factors can trigger chronic inflammatory responses and consequently augment chronic inflammatory conditions to promote tumor growth. The connection between senescence and aging was initially based on the observation that senescent cells accumulate in aged tissue. The use of transgenic models has enabled the detection of senescent cells systematically in many age-related disorders. Studies have demonstrated that senescent cells play an adverse role in age-related disorders.
By leveraging our immunology expertise, our Company is developing potentially transformative immunotherapy candidates that use inflammasome modulation and senescent cell reduction to address chronic inflammation linked especially to age-related diseases. Our approach is to develop immunotherapies that reduce or eliminate the main drivers of chronic inflammation by addressing the underlying development and sustainment of these processes.
Using our drug discovery platforms, we have successfully developed over 55 molecules, some of which can be administered by subcutaneous injection as well as used in adoptive cell therapy approaches. These modular and tunable technologies have allowed us to generate a novel pipeline of internally developed product candidates capable of activating and targeting desired immune responses and blocking unwanted immunosuppressive activities.
HCW9218: Clinical-Stage Bifunctional Molecule
Advances in immuno-stimulatory and anti-immunosuppressive therapeutics have revolutionized treatment of multiple diseases. However, novel immunotherapeutics with these dual functions are not frequently constructed. We have used our TOBITM platform to construct one of our product candidates, HCW9218, designed with two functionalities. It rejuvenates the immune system to reduce senescence, and it captures transforming growth factor-β (“TGF-β”) to neutralize its immunosuppressive activity. HCW9218 is a novel immunotherapeutic that is a heterodimeric, bifunctional fusion protein complex comprising extracellular domains of the human TGF-β receptor II, as TGF-β trap for TGF-β neutralization, and a human interleukin (IL)-15/IL-15 receptor α complex for immune-cell stimulation. For further detail, see our publication entitled “Bifunctional TGF-β trap/IL-15 Protein Complex Elicits Potent NK Cell and CD8+ T Cell Immunity against Solid Tumors”, Molecular Therapy 2021 Oct 6;29(10):2949-2962.
HCW9218 potently activates NK cells and CD8+ T cells in vitro and in vivo to promote their proliferative and metabolic activities and enhances their cytotoxicity against disease targets. This fusion complex also exhibited TGF-β neutralizing activity in vitro and sequestered plasma TGF-β in mice and non-human primates. Preliminary human data readouts from our Phase 1 clinical trials to evaluate HCW9218 in solid tumors provided encouraging data consistent with our preclinical findings. For further details, see our published scientific paper entitled “Immunotherapeutic HCW9218 Augments Anti-tumor Activity of Chemotherapy via NK Cell Mediated Reduction of Therapy Induced Senescent Cells,” Molecular Therapy (2022) 30:1171-1187.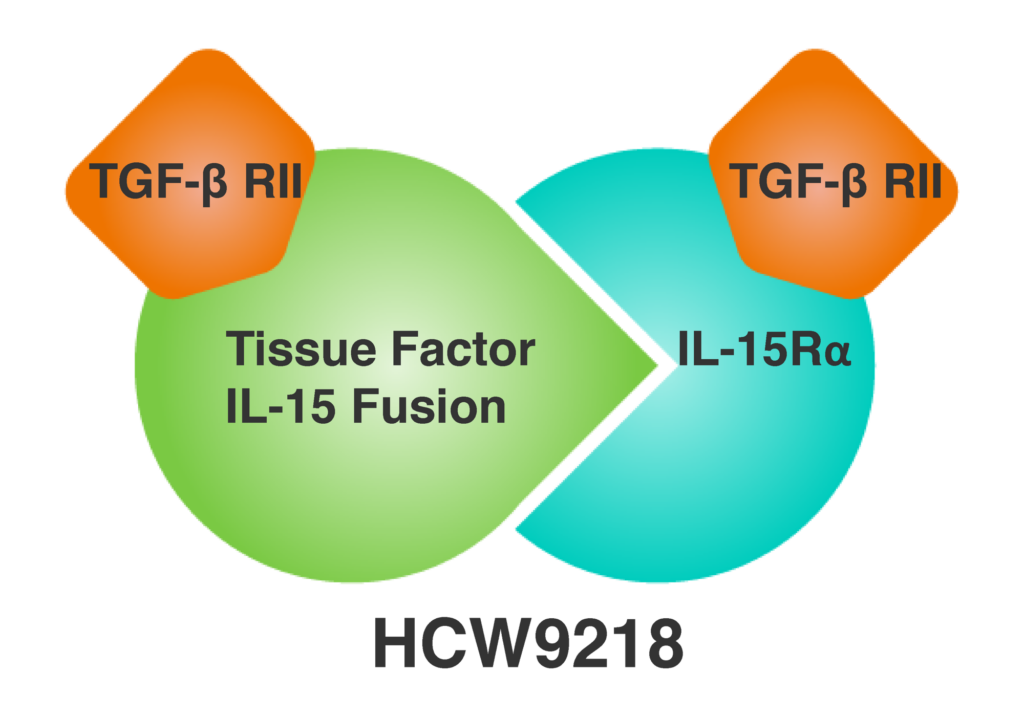
HCW9218: Treatments to Improve Quality of Life
HCW Biologics holds the perpetual, exclusive worldwide license to develop immunotherapeutic treatments based on HCW9218 for age-related diseases other than cancer. HCW9218 may have much broader therapeutic potential beyond cancer to treat other age-related diseases and conditions. This is based on its ability to promote cell-mediated mechanisms to reduce senescent cells and alleviate the proinflammatory factors they secrete. Scientists from the company hypothesized that HCW9218 is an immunotherapeutic agent that rejuvenates a dysfunctional immune system and neutralizes TGF-β and can act as an effective senescent-cell reducing and senomorphic drug.
For future investigative studies, we will rely on the recommended Phase 2 dose determined in our completed Phase 1 clinical trials for solid tumors. The Company is currently assessing a number of indications broadly categorized as “quality of life” – including aesthetics for deep wrinkles and senile lentigo – in search of candidates to advance in clinical development.
The company published a pivotal scientific paper in Aging Cell entitled, “Immunotherapeutic approach to reduce senescent cells and alleviate senescence-associated secretary phenotype in mice,” with Dr. Hing C. Wong, our Founder and CEO, as lead and corresponding author. For further details, see Shrestha N. et al., “Immunotherapeutic Approach to Reduce Senescent Cells and Alleviate Senescence-Associated Secretory Phenotype in Mice,” Aging Cell 2023 Mar 26:e13806.
HCW9302: Treg Expansion
HCW Biologics holds the perpetual, exclusive worldwide license to develop immunotherapeutic treatments based on HCW9302 for all disease indications, with no restrictions.
Our lead molecule, HCW9302, is an IL-2-based immunotherapeutic agent, designed to stimulate regulatory T cells (“Treg cells”) to suppress the activity of inflammasome-bearing cells and inflammatory factors that drives senescence. Treg cells are essential mediators of peripheral tolerance and the global immunoregulatory potential in hosts to self and non-self-antigens. Treg cells achieve this immunoregulatory control through multiple suppressive mechanisms. Alternations in Treg cell development, homeostasis or function can predispose these cells to affect a variety of disease conditions including allergy, autoimmunity, graft rejection, cancer, and response to immunotherapies.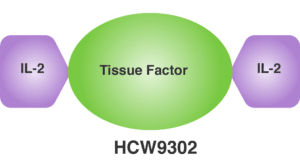
HCW9302: Autoimmune and Pro-Inflammatory Indications
In the development of HCW9302, our approach has been to deactivate inflammasome pathways in monocytes and macrophages through the immunosuppressive activities of Treg cells induced by our immunomodulator molecules. This approach does not rely on inhibiting specific inflammasome components but rather utilizes natural processes of the immune system to attenuate and rebalance chronic self-perpetuating pro-inflammatory responses. In relevant animal models, we have observed encouraging results using HCW9302 to activate and expand Treg cells for treatment of atherosclerosis and diabetes.
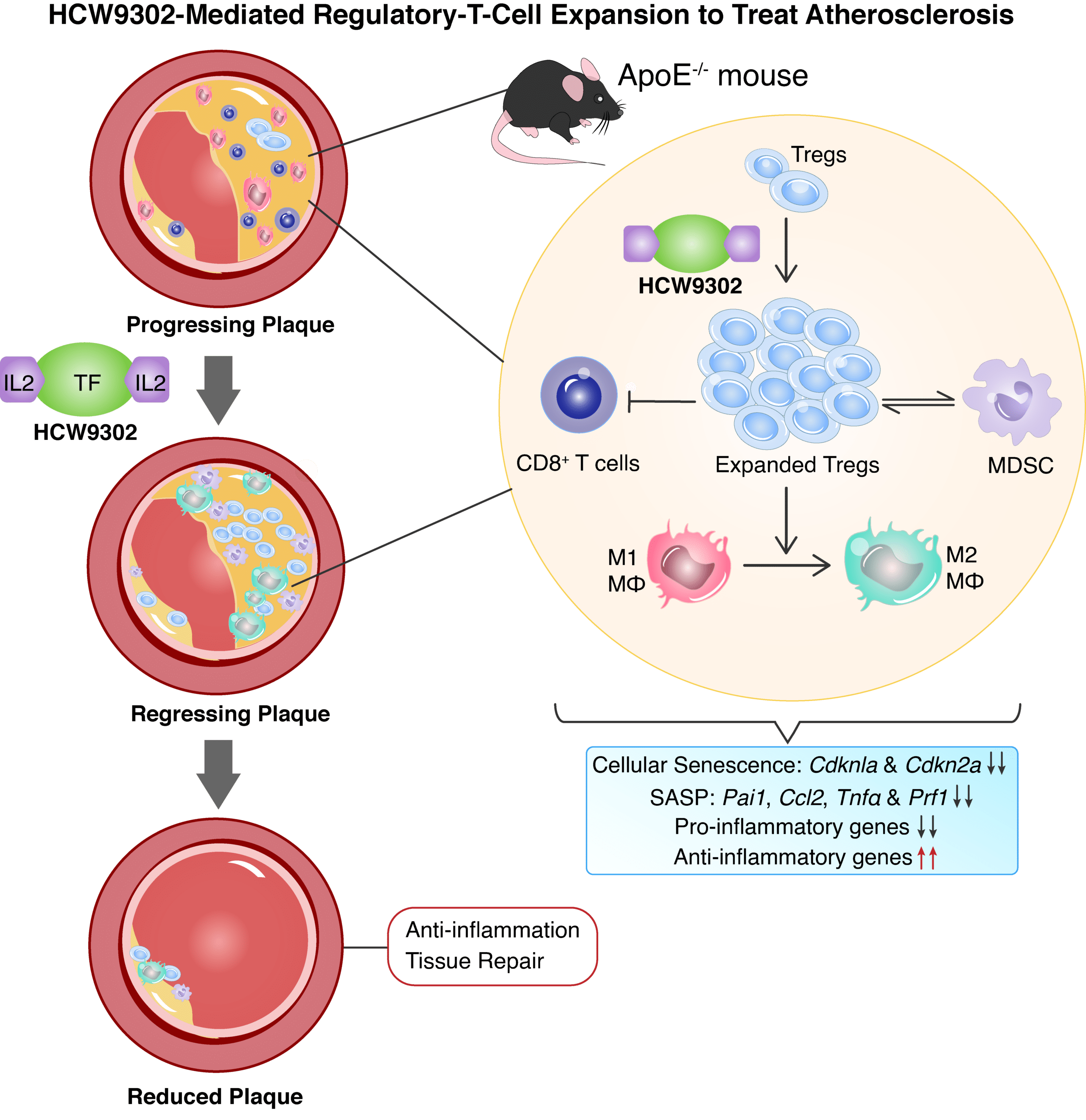
Our data suggest that HCW9302 functions as a potent agent to stimulate Treg cells that suppress the activity of inflammasome-bearing cells and inflammatory factors. We also observed a long serum half-life compared with rhIL-2 which contribute to the ability of subcutaneously administered HCW9302 to activate and expand Treg cells in mice in a well-tolerated dose range without activating proatherogenic CD4+ T cells. This finding also suggests that greater CD25-mediated Treg activation may be superior to “mutein”-based strategies to prevent or diminish IL-2 binding to IL-2Rβγ on effector cells. For further details, see our publication entitled “A Novel Interleukin-2-Based Fusion Molecule, HCW9302, Differentially Promotes Regulatory T Cell Expansion to Treat Atherosclerosis in Mice,” Frontiers in Immunology 2023 Jan 25;14:1114802.
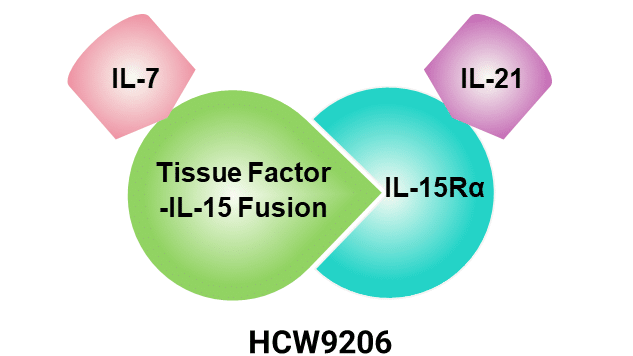
HCW9206: Potent Immune Response Recovery for Cancer and Infectious Diseases
HCW Biologics holds the perpetual, exclusive worldwide license to develop immunotherapeutic treatments based on HCW9206 for all disease indications, with some commercially-reasonable restrictions for cancer indications.
Our lead molecule, HCW9206 is a fusion molecule incorporating IL-7, IL-15, and IL-21. It represents a pioneering approach in immunotherapy against cancer and aging-related diseases. IL-7, critical for T cell development and survival, holds promise in conditions like cancer and HIV by supporting the survival of both naive and memory T cells, potentially aiding immune recovery. IL-15, pivotal for activating T cells and NK cells, shows potential in cancer treatment by enhancing cytotoxicity and maintaining memory CD8+ T cells, thereby bolstering anti-tumor responses. IL-21, produced by activated T and NK cells, plays a versatile role in immune function, promoting T and B cell proliferation, enhancing NK cell activity, and influencing dendritic cells to enhance immune responses against tumors and chronic and acute infections.
HCW9206 synergistically harnesses these cytokines’ immunostimulatory properties to augment T cell proliferation, enhance NK cell cytotoxicity, and improve overall immune surveillance against pathogens or tumors. Injectable HCW9206 formulations offer advantages in precision and controlled release kinetics over infusion, potentially optimizing immune activation over extended periods. Future clinical trials will evaluate the safety, efficacy, and optimal dosing of HCW9206 across various diseases, including vaccine efficacy, oncology and infectious diseases, paving the way for innovative approaches to immune modulation and disease treatment.
TRBC Platform-Based Molecules
By employing our TRBC platform technology, we engineered fusion molecules which have capabilities to stimulate immune functions while also modulating various signaling pathways. These compounds have the potential to unlock transformative immunotherapy candidates by utilizing inflammasome modulation and senescent cell clearance to combat chronic inflammation associated with age-related diseases. They are product candidates designed to precisely activate and target immune responses while inhibiting undesirable immunosuppressive actions and can be administered via subcutaneous injection. Their versatility opens up a wide range of potential applications across various indications, including hematologic and solid tumors, virally infected cells, autoimmune diseases, and cellular senescence diseases associated with ageing.
The TRBC molecules could be classified into three classes.